FOR IMMEDIATE RELEASE
FOR IMMEDIATE RELEASE
FOR MORE INFORMATION:
Contact: Christopher Sellwood
Phone: (612) 616-4786
Email: csellwood@curemedical.com
Cure Dextra® Closed System Offers a New Way to Advance
Newport Beach, CA, January 1, 2021 – Cure Medical LLC introduced the Cure Dextra® Closed System serving a broad market of able bodied catheter users and those with limited hand dexterity.
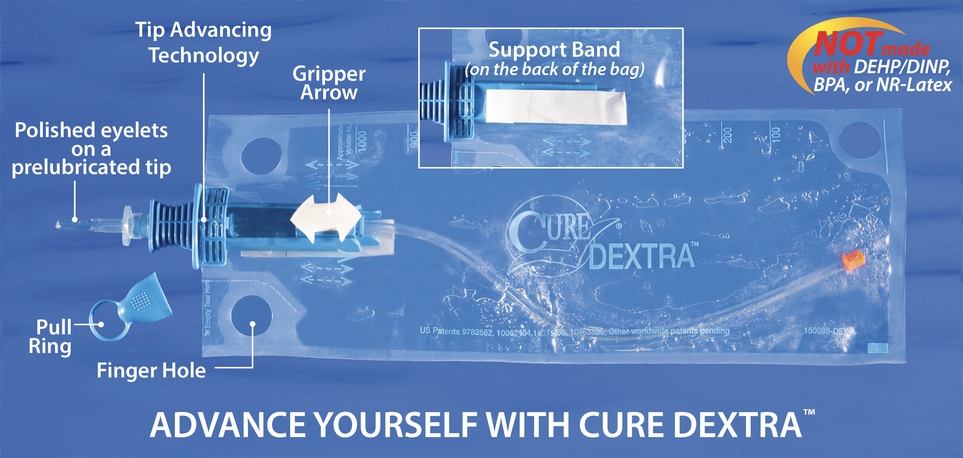
Catheter users value Cure Dextra® proprietary attributes intended to facilitate use, increase independence, and reduce risk of infections. A Support Band enables controlled use. Tip Advancing Technology moves the pre-lubricated catheter from the 1000 ml collection bag into the body without contact. Sterile catheterization is possible without gloves. A Gripper Arrow facilitates advancing the catheter with each forward stroke.
Like all Cure Medical intermittent catheters, the Cure Dextra® features polished eyelets for increased comfort and is made of quality materials – not made with DEHP*/DINP, BPA or Natural Rubber Latex.
Cure Medical has made an unsurpassed Cure Commitment to donate ten percent of net income to research in pursuit of a cure for spinal cord injuries and central nervous system disorders. As a result, it is possible to provide customers with a unique, future benefit along with the immediate, positive experience associated with use of the Cure Dextra® Closed System.
Cure Medical supports partner suppliers with free samples, co-op advertising, and lead-generating marketing tools. For more information, call (612) 616-47861.
*https://www.p65warnings.ca.gov
# # #
About Cure Medical LLC
Founded in 2007 as a means for sustained funding of medical research, Cure Medical LLC develops traditional and innovative catheters for distribution through a network of healthcare product suppliers.
Cure Medical, LLC, 3471 Via Lido, Suite 211, Newport Beach, CA 92663 • Phone: (800) 570-1778 • Phone: (949) 723-0364 • Fax: (949) 723-0564 • Web: www.curemedical-dextra.com