Cure Medical appreciates all medical professionals serving the rehabilitation and urologic community. Like you, we endeavor to enhance the quality of life for those affected by disability or urological conditions which require use of intermittent catheters for bladder health. To that end, we seek excellence in catheter design and manufacturing.
Cure Medical appreciates all medical professionals serving the rehabilitation and urologic community. Like you, we endeavor to enhance the quality of life for those affected by disability or urological conditions which require use of intermittent catheters for bladder health. To that end, we seek excellence in catheter design and manufacturing.
Medical professionals are invited to request complimentary samples of our catheters and to subscribe to the monthly UROLOGY INSIGHTS eNewsletter.
UROLOGY INSIGHTS e-Newsletter Archives
UROLOGY INSIGHTS is written by Melissa Fulton, BSN, MSN, FNP, APRN-C as an independent consultant. Content is not a reflection of Cure Medical nor is it a substitute for your own medical training and judgment.
PATIENT BENEFITS
Cure Medical designs and manufacturers catheters with utmost considerations for patient safety, comfort, and ease of use. User-driven innovations are evident from packaging to product features. Additionally, only Cure Medical offers the Cure Commitment to sustained support of medical research.
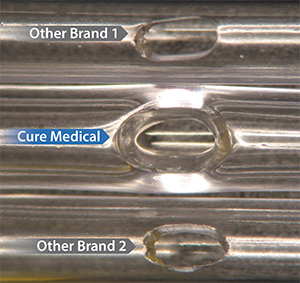
Polished Eyelets
Some other catheter manufacturers use a low cost production technique to ‘cold punch’ eyelets. This can produce rough edges (see Other Brand 1 and Other Brand 2). Some other manufacturers ‘cold punch’ eyelets on their standard line of catheters and only polish the eyelets on their premium catheters.
In contrast to both of these practices, Cure Medical Catheters have smooth, fire polished eyelets that help enable comfortable use. This standard for manufacturing excellence has a benefit that can not only be seen – it can be felt by the catheter user!
User-Driven Innovations
Cure Medical continuously pursues product innovations to help ensure a positive user experience. Examples include easy-open twist, tear, or peel packaging plus dual openings on some product pouches.
Some product features facilitate proper use – such as the control stripe on Coude tip catheters to help guide insertion. Other product features enable ease of use even with limited dexterity or weak pinch strength – such as the Support Band and Gripper Arrow on the new Cure Dextra™ Closed System.
No Harmful Chemicals or Carcinogens
Some other manufacturers of intermittent catheters use Di(2-ethylhexyl)phthalate (DEHP) or Diisononyl Phthalate (DINP) as an economical way to make catheters flexible. These phthalates are included in a published list* of chemicals known to cause cancer and reproductive harm according to the state of California. As a result of Proposition 65, manufacturers that continue to use phthalates in their catheters must include a warning label on their product packaging, catalog, and website.
In contrast to a warning, Cure Medical offers an assurance that our catheters are not made with DEHP/DINP, BPA or Natural Rubber Latex.
http://www.p65warnings.ca.gov/